5.3.1 Chlorination
Chlorine is one of the most practical and widely used disinfectants because it destroys target organisms by oxidising the cellular material of bacteria. Chlorine is available in various forms, such as liquid, solid or gas. Liquid sodium hypochlorite (NaOCl, also known as bleach) and solid calcium hypochlorite tablets are the most common forms of chlorine used for small systems because they are less hazardous than chlorine gas.
Chlorine reacts with various components in wastewater, such as hydrogen sulphide, ferrous iron, manganese, and nitrite, before reacting with pathogens. Ammonia (NH3) – present in all wastewater, is the second level of reaction with chlorine. It combines with chlorine to form chloramine which acts as a disinfectant. Finally, the chlorine will react with organic compounds in the wastewater and form chloro-organic compounds which have also a slight disinfection capability. The chlorine demand refers to the amount of chlorine that is consumed by the various materials that react with it before it can effectively neutralize pathogens.
Chlorine in combined forms that have disinfecting properties (chloramine, chloro-organic compounds) plus any free chlorine is defined as chlorine residual. This residual is the portion of the chlorine dosage which will realise the disinfection. Chlorine dosage is therefore the sum of chlorine demand and chlorine residual.
Chlorine Dosage = Chlorine Demand + Chlorine Residual
The Chlorine residual remains in the effluent after the initial disinfection has occurred but will decay with time and is the key parameter to be monitored. The chlorine dosage should be set to achieve a target residual. The recommended value for chlorine residual varies in available guidelines, but it is typically advisable to aim for 0.2 mg/L. To measure chlorine residual, DPD 1 (a chemical reagent) or test strips can be utilized.
The chlorine dosage and process efficiency are influenced by several factors. Amongst others, effective pre-treatment will lower the suspended solids and organic content of the wastewater, which lowers the chlorine demand and results in a reduced chlorine dosage. Some values are shown in the following table.
| Calcium Hypochlorite | Septic Tank Effluent | Biological Treatment Effluent | Sand Filter Effluent |
|---|---|---|---|
| pH 6 | 35 – 50 mg/L | 15 – 30 mg/L | 2 – 10 mg/L |
| pH 7 | 40 – 55 mg/L | 20 – 35 mg/L | 10 – 20 mg/L |
| pH 8 | 50 – 65 mg/L | 30 – 45 mg/L | 20 – 35 mg/L |
Note: Contact time is 1 hour at average flow and temperature 20°C. Increase contact time to 2 hours at 10°C and 8 hours at 5°C for comparable efficiency.
The table also shows the impact of pH levels. Chlorine reacts with water to form hypochlorous acid (HOCl) and hydrochloric acid (OCl–). The pH value will control if either HOCl or OCl– will be formed. Hypochlorous acid, which is specially formed at lower pH, has a greater disinfection potential than hydrochloric acids. Therefore, chlorination is most effective at a pH <8.0. Lower or neutral pH values result in more efficient use of chlorine as a disinfectant.

Adequate contact time is critical to ensure that the applied chlorine has sufficient time to provide effective kill/inactivation of pathogenic organisms that may be present in the water. Contact time is typically achieved in a storage or contact tank following the chlorine application. Baffling within the tank or basin helps minimize short-circuiting and aids in mixing. The recommended length-to-width ratio is 40:1, and the unit is recommended to be designed for a 30-minutes of contact time at maximum month average flow or at least 15 minutes of contact time at peak hourly flow. Generally, the longer the contact or detention time, the higher the degree of disinfection.
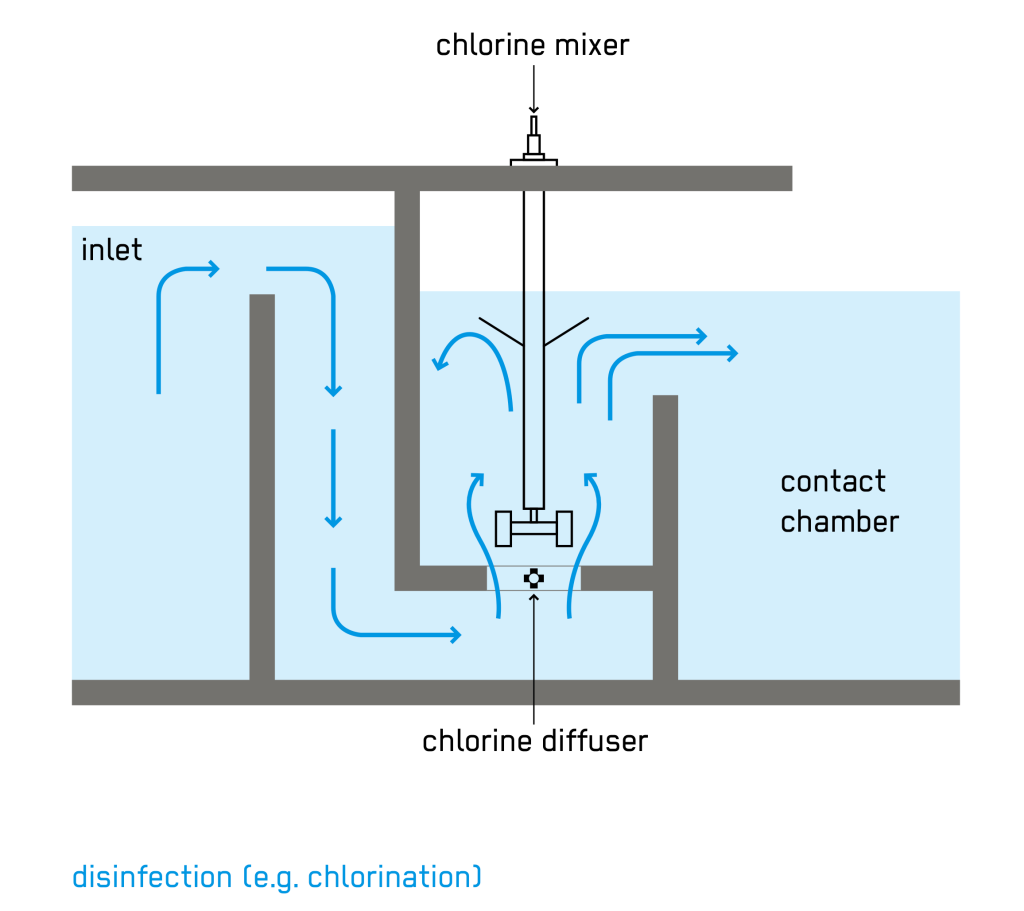
(Source: Emersan Compendium)
The required contact time is related to the ambient temperature. Higher temperatures will result in more efficient disinfection. Lower temperatures need to be compensated by longer retention times and higher chlorine dosages. E.g. the standard contact time of 30 minutes at 25°C must be doubled with a 10°C temperature drop.
Overall, chlorination shows a reliable efficiency in killing viruses and bacteria cells, while helminths and protozoa are rather resistant to chlorine. If it is determined that the effluent is rich in cysts and spores, an alternative disinfection treatment such as UV radiation should be chosen.
When effluent is intended to be discharged into streams, rivers, and lakes which provide habitat for wildlife and plant life, dechlorination (the removal of chlorine) should be considered. As chlorine is toxic, it can otherwise harm the aquatic life.
After disinfecting wastewater with chlorine, residual chlorine may be present in the effluent for a few hours. However, chlorine alone can be harmful to aquatic life. To minimise the impact, it is often necessary to dechlorinate the wastewater. This process removes both the free and combined chlorine residuals, reducing their toxicity before the wastewater is discharged. Common chemicals used for dechlorination are sulphur dioxide, sodium bisulphites, and sodium metabisulfite. Additionally, activated carbon can also be used for this purpose.
The table presented below outlines the benefits and limitations of chlorination.
| Benefits | Limitations |
|---|---|
| Reliable and effective against a wide spectrum of pathogenic organisms | Chlorine residual is toxic to aquatic life. Dechlorination may be required even when low concentrations of chlorine are used. |
| More cost-effective than UV or ozone disinfection | Highly corrosive and toxic. Increased safety for storage, shipping, and handling (eye and hand protection). |
| Chlorine residual can prolong disinfection even after initial treatment and can be measured to evaluate the effectiveness | Reacts with certain types of organic matter, creating hazardous compounds |
| Dosing rates are flexible and can be controlled easily | Chlorine residuals are unstable in the presence of high concentrations of chlorine-demanding materials (BOD). Effluent with high BOD may require higher chlorine doses for adequate disinfection. |
| Low efficiency regarding protozoa and helminths eggs |
For further information, please click on the Materials tab at the top of the page.
Further Reading:
- Basic Chemistry of Chlorination (Link)
- Wastewater Technology Factsheet – Chlorine Disinfection (EPA) (Link)
- Wastewater Treatment Plant Operator Certification Training – Module 5: Disinfection and Chlorination (Link)
- Chlorine for Water Treatment (OXFAM) (Link)
- Onsite Wastewater Treatment Systems Manual (USEPA) (Link)
