6.4.3 Hydrogen Sulphide
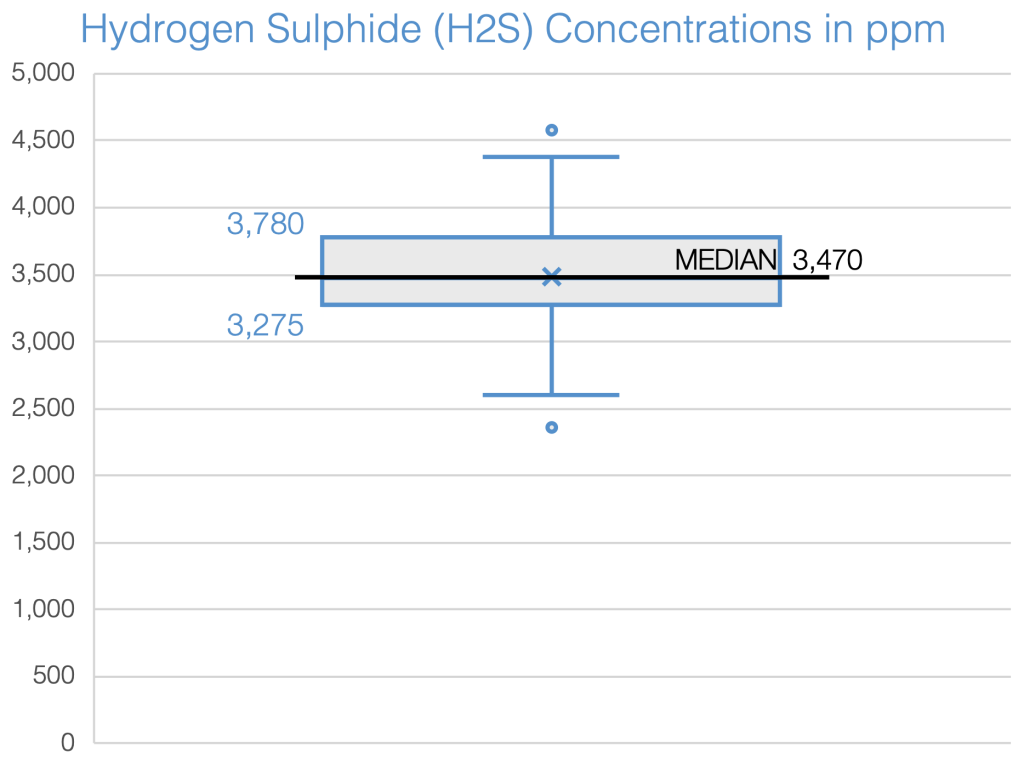
In 2019, an analysis was conducted on the characteristics of biogas for 19 biogas digesters, which included measuring the concentration of hydrogen sulphide in parts per million (ppm). The findings showed that the typical concentration observed fell within the range of 3,200 to 3,800 ppm, as indicated in the boxplot.
Hydrogen sulphide (H2S) is a common and hazardous component of biogas. It is produced by anaerobic microorganisms that degrade sulphur-containing proteins or inorganic sulphates in organic matter. H2S is a colourless gas with a characteristic “rotten egg” smell at low concentrations. When H2S reacts with water, it forms sulfuric acid, which is highly corrosive and can damage equipment. Moreover, when H2S burns, it generates sulphur oxides (SOx), which are also harmful to the environment and human health.
Out of 22 biogas plants inspected, it was found that 82% of the biogas stoves were in fair condition, while 14% were in poor condition. The prime reason for this was corrosion on the end-use equipment, which was caused by the combustion of H2S.

H2S is a harmful gas that can cause health problems and damage equipment. To ensure the safe use of biogas, it is crucial to remove hydrogen sulphide (H2S) from the gas before it is supplied to stoves or other appliances. This can be achieved by installing a desulphurisation unit (scrubber) in the gas supply line. The desulphurisation unit typically consists of a container filled with a reactive agent that reacts with H2S to remove it from the gas.

One commonly used agent for scrubbing is iron oxide, which can be sourced from natural soils or certain ores containing iron as the primary element or as part of an alloy. The cost of the absorbent is typically around 0.20 to 0.80 USD cents per kilogram, and 1 kilogram can treat approximately 200m3 of gas.
Notably, steel wool with a grade of 000 is the least effective in H2S removal, with a reported removal rate of around 40% (Link).
Since iron oxide was not readily available during the assessment in Bangladesh, metal shavings and iron dust were tested as reagents, but they did not perform well. Even though the initial removal rates were 99%, they quickly declined, reaching 0 – 55% after only a few days of operation.
Therefore, selecting appropriate desulfurization materials and monitoring their performance is important to maintain efficient H2S removal in biogas systems. If the reagent materials are not easily available, some research and experimentation may be needed, by trying out different reagents such as activated baking soda, lime, or other alkaline substances, char or activated carbon, clay, and water scrubbing. Or a combination of different materials.
Regular maintenance and replacement of the desulfurization units are also necessary to ensure optimal performance.
Given the challenges with sourcing specific reagents, conducting further literature research and experimentation is advisable to explore different options. Some potential alternatives for H2S removal in biogas systems include baking soda, lime, or other alkaline materials, char or activated carbon, clay, and water scrubbing. Combining different materials will yield improved results.
